Over- and underdrainage
- the most common “side effects” of shunt systems
A look back at the last 70 years of over- and underdrainage in shunts
Over- and underdrainage are inextricably linked to the normal physical functioning of shunts. For instance, neither problem is the result of malfunctions caused by negligence or a lack of understanding. Rather, such problems are an inevitable result of using a shunt to create an artificial, unphysiological link between body cavities that are usually separate from one-another. That is why it is so important to be familiar with the basic principles of both phenomena in terms of physics, meaning that they can be treated with the right combination of treatment and technology.
show more"A shunt is like a horse harnessed to a broken-down car."
There are many different ways in which hydrocephalus can manifest itself and it has many different causes. Although people have been aware of the symptoms since ancient times, most of the causes, to use scientific language, are “not particularly well understood”. There are even some that are completely unknown. It is difficult to tackle causes that are unknown or not understood. At the same time, this condition is far from rare and can affect any age group.
Even today, it is only treated symptomatically. This involves reducing the dangerous overpressure in the patient’s skull using a valve and draining it into another suitable body cavity (often in the abdomen).
SHUNT SYSTEMS
A shunt system usually consists of a (proximal) ventricular catheter, a (frontal or occipital) valve to regulate the opening pressure and a (distal) catheter for draining it into a particular body cavity (the right atrium of the heart or the abdomen). A shunt system like this can also be equipped with a reservoir (burr hole reservoir or prechamber).
The first shunt was implanted almost exactly 70 years ago (in 1949) in Philadelphia by the neurosurgeons Spitz and Nulsen. It was so basic and prone to failure that, as far as we know, it only aided a single patient for a short time. Six years later, the valve improved significantly by the locksmith, John Holter, meaning that most people view the “shunt therapy era” as only beginning in 1955. The famous story of how John Holt tried desperately to fight for the life of his newborn son (ultimately in vain, sadly), who was suffering from hydrocephalus is set out in the following, very well-written, account: HOLTER’S BRAIN DRAIN.
Since then, the basic principle of “shunting” has remained the same, which is unusual in the field of medicine. However, from a purely technological standpoint, there have been many significant improvements. Today, high-quality shunts are made from durable, biocompatible materials to ensure that they do not break, corrode or trigger a rejection reaction. Antibacterial catheters can reduce rates of infection to less than 5% (Ritz1, 2007; Parker2, 2015) and opening pressures can be adjusted to suit the specific needs of the patient. Modern shunts like this allow many patients to lead a long life with barely any restrictions, which really does represent a major step forward.
However, despite fantastic modern shunts, the continued lack of knowledge about the causes of hydrocephalus has lead to problems when treating the condition.
To illustrate things better, perhaps it would be useful to compare the shunt with a horse tied to a car that has a faulty engine. Even though the no-one really knows the reason for the engine damage, the car will move again (even if it is a bit slower) and will therefore be able to reach its original destination. It does not matter whether the problem has been caused by a tear in the fuel line, a broken engine mount or a defective spark plug, as the method will almost always work for the time being. You just have to follow a few new rules: e.g. feeding the horse regularly and giving it breaks. AND: Now new, completely different things can suddenly go wrong. The horse could simply gallop off down a rough track, pulling the car in the wrong direction or even drag it into the ditch. The driver, who before only had to know about his car, now has to be able to drive the horse and learn to understand it.
For example, a shunt can get blocked over time, and, even today, implanting a new one is often the only available solution. So-called “siphoning” is another particularly unpleasant, but unavoidable side-effect: the laws of physics mean that when a person is standing, a high level of suction (or negative pressure) is produced in the ventricles, between the head and abdomen, due to the fact that gravity causes the liquid in the catheter to be drawn downwards. This suction poses as great a danger to the brain as the original overpressure (hydrocephalus) can give rise to brain haemorrhages. (The following article provides more detailed information on this phenomenon: "Foundations of fluid mechanics, or of beakers, bottles and people?"
It is not possible to stave off this side effect using a normal shunt: special techniques are required to counteract it.
Underdrainage is therefore inextricably linked to the normal physical functioning of the shunt, as is overdrainage, which is particularly dangerous. For instance, neither problem is the result of malfunctions caused by negligence or a lack of understanding. Rather, such problems are an inevitable result of using a shunt to create an artificial, unphysiological link between body cavities that are usually separate from one-another.
It is almost impossible to prevent either overdrainage or underdrainage when implanting a single differential pressure valve. This procedure is just as common today as was in the past. The only thing the responsible doctor can do is to cleverly configure the valve in a way that constitutes a "clinically acceptable" compromise between these two issues, under the circumstances. Simply implanting another valve to counteract the physical forces responsible for overdrainage, and the occasionally dramatic effects it has on the patient, is something that is important to avoid. This will be explained in the section below.
Underdrainage means that too little CSF is being discharged through the shunt. The pathological overpressure in the ventricles goes back to roughly the way it was before or is not sufficiently reduced. Some or all of the original symptoms of hydrocephalus, including headaches, vertigo and nausea will then return. Children may also experience changes in their facial expression (sunset eyes, papilloedema) and a gradual increase in head circumference. The cardinal symptoms that affect normal pressure hydrocephalus (NPH) patients are incontinence, symptoms of confusion similar to dementia and the typical gait abnormalities, causing the patient to take small steps. These outwardly-visible symptoms will be constant: to be more specific, they will occur regardless of whether the patient is standing or lying down.

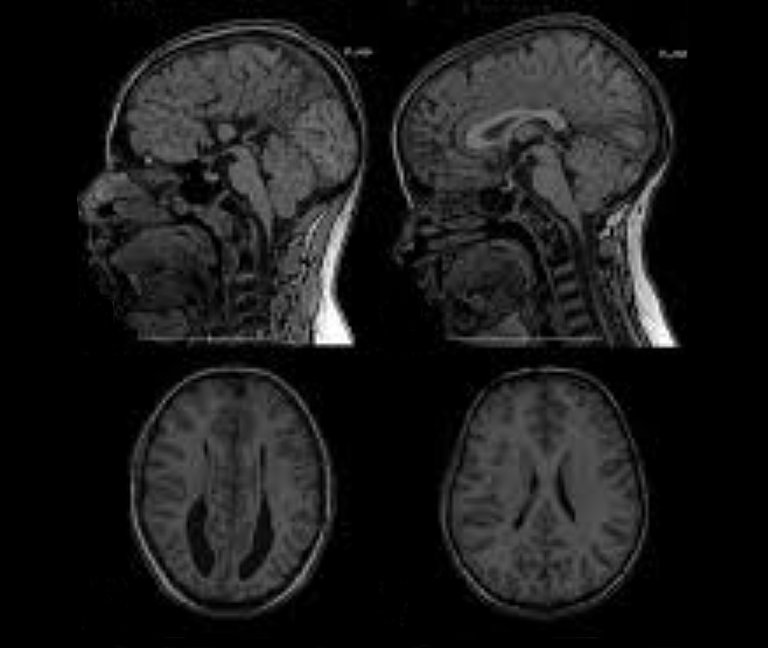
Sometimes, the MRI or CT imaging will show that the overpressure caused by underdrainage is leading the ventricles to dilate, compressing the brain tissue and pushing it against the cranial wall from the inside. This destroys nerve cells and causes them to die-off. The process takes place slowly, over the course of weeks and months, depending on the level of overpressure. Nevertheless, in the long term it is irreversible, due to the fact that the nerve cells cannot be regenerated.
Underdrainage can easily be explained by valves in which the flow-resistance is too high or the opening pressure is too high: they are simply not fulfilling their intended therapeutic function. In such a case, if an adjustable valve has been implanted, the first countermeasure should of course be opening it up. This should be done while the amount of overpressure is still low, so that the CFS to be drained early on. If this does not lead to any improvement, the valve may be blocked, meaning that a revision will need to be carried out.
Overdrainage means that too much CSF is being discharged, leading to low intraventricular pressure and sometimes even suction in the ventricles.
The outwardly-visible acute symptoms are largely similar to those associated with underdrainage and include headaches, vertigo and nausea. At first, it is difficult to tell the difference between the two types of complication.
However, in cases of overdrainage, unlike with underdrainage, the imaging may show that the ventricle has shrunk or collapsed completely – a condition known as “slit ventricle syndrome”.
Slit ventricles do not have any pathological significance per se. The term simply refers to the width of the cerebral ventricles that narrow into slits due to overdrainage. However, if, for example, the CT or MRI scan appears to show this issue it will confirm a suspected diagnosis of "overdrainage".
INTRAVENTRICULAR PRESSURE
Intraventricular Pressure (IVP) is the differential pressure relative to the external atmospheric pressure. “Negative IVP” or suction therefore occurs when the pressure in the ventricles is lower than the atmospheric pressure.
Overdrainage can put brain tissue under significant pressure. However, this is not caused by compression, but by tensile stress, which pulls the surface of the cerebral cortex away from the cranial wall, towards the ventricle. There have even been documented cases in which this suction has caused the brain stem or cerebellum to move, bending the very narrow aqueduct (an important channel that connects the third and fourth ventricle).
Nevertheless, these imaging techniques often do not immediately lead to such a clear diagnosis, if they even lead to any diagnosis at all. In fact, they often appear unremarkable: the aforementioned morphological changes to the ventricle and the brain take time to develop. Naturally, this also depends on the severity of the overdrainage and the compliance of the brain.
Compliance
“Compliance” is an important term in the field of hydrocephalus therapy. This is not only typical in the brain, but also affects the entire central nervous system (CNS), i.e. the cranial and spinal cavities. Almost all of the space in these areas is taken up by brain tissue and the spinal cord (approximately 1,400 ml). They also contain arterial and venal blood (approximately 150 ml in total, at a ratio of 1:2), as well as CSF (approximately 150 ml).

To give a clearer description, compliance reflects something like the “softness” or “elasticity” of the entire contents of the cranial and spinal cavities. This elasticity is a type of “pseudo-elasticity”, due to the fact that the elements contained in the cranial/spinal cavity, such as blood and CSF, are inherently incompressible, or, in other words, rigid. Compliance comes about due to the fact that with every new “mass”, e.g. a buildup of excess CSF (hydrocephalus), a bleed (haematoma) or a growth (cancer, cysts or swelling), venal blood is essentially displaced from the rigid space and forced out into the body. Therefore, if compliance is normal, such masses will only cause a slight increase in pressure within the skull (intracranial pressure or ICP) to begin with, which is why they are often associated with a so-called "pressure reserve capacity”. However, when the mass becomes too large the reserve capacity quickly gets used up and the ICP increases sharply. However, masses that are too large are not the only reason for reduced compliance. In fact, this can also be caused by a pathological hardening of the tissue and blood vessels, particularly the veins (sclerosis). Measuring compliance can also be said to have “diagnostic value”, as it makes it possible to find out some information regarding the size of the mass, as well as the state of the tissue and blood vessels.
The precise medical definition of compliance in the form of a pressure to volume ratio (C = dV/dP) is set out in the following pressure-volume curve:

A certain physiological (natural/healthy) mass in the cranial cavity is caused by the wave-like inflow of arterial blood. The following diagram shows how the amplitude (i.e. the level) of the constant ICP pulse waves depends on the level of compliance: a high ICP wave amplitude shows a reduced level of compliance.

Although only a small percentage of the total CSF is contained in the bony spinal canal, this means it contains a greater amount of venal blood. The dural sac, which contains the spinal CSF, is flexible and expands significantly when the person stands, due to the CSF sinking down from the skull. The spinal canal probably accounts for more than 50% of total compliance.
Overdrainage is not as easy to explain as underdrainage. How can such a large amount of suction be produced just by implanting a shunt, i.e. artificially linking the ventricle with the abdomen (as is usually the case)? The only place in the human body in which there is a certain level of moderate negative pressure (suction) is the pleural gap (p = approx. - 5cmH2O), but this is rarely considered for use as an outflow for drainage anymore. In the past, there was also speculation about a “dynamic pump mechanism” e.g. in crying children. However, this could not be confirmed.
However so-called hydrostatic pressure (HSP) could provide a satisfactory and plausible explanation. This only occurs when the patient is in a standing position and is caused by the weight of the column of liquid in the shunt (i.e. particularly in the long peritoneal catheter). At the bottom end of the catheter this weight becomes noticeable due to the weight pressure placed on the column of liquid. However, there is suction at the upper end of the catheter in the form of hydrostatic pressure (HSP) or hydrostatic suction, depending on the reference point. Without a valve, the column of liquid would simply flow through the open catheters at the top and bottom, with the process being driven by the weight of the liquid. In other words: if the patient stands up straight and the ventricles above are connected to the abdomen below via the long catheter, they will "want" to empty all the fluid out. Another way to describe this to say the ventricles are "sucked dry", which is why the term "siphoning" is often used to describe this effect. However, this extreme scenario would only occur if external air were able to flow through the ventricle. However, given that the ventricles are usually closed, the CSF only keeps draining away until the hydrostatic suction is balanced out by the tensile stress that occurs in the brain tissue ("counter suction"). The degree to which this will occur in a specific situation depends, inter alia, on the elasticity of the tissue (compliance) and the amount of suction.
Hydrostatic pressure in cmH2O
To use technical jargon, pressure in the CFS is measured using the non-SI unit "cmH2O" ("SI" stands for Système international d'unités or the "International System of Units"). This unit is otherwise known as the "centimetre of water". This is a type of so-called "differential pressure", which is used to describe the difference in pressure between the cranial cavity and the external air pressure.
cmH2O is defined as the hydrostatic pressure exerted by a one-centimetre-high column of water, in which the density of the water is precisely one gram per cubic centimetre. It is expressed differently to the unit "Pa", which is normally used to measure pressure. Unlike a "Pascal", which is defined as the force of one Newton applied to an area of one cubic metre, the cmH2O is a unit that does not have to refer to an effective surface area. It only refers to a one-dimensional measure of length, namely the height of the column of water exerting force on the relevant pressure point. This goes against our intuitive understanding of pressure, however, in doing so, it makes the effect of hydrostatic pressure (i.e. the pressure in a medium, such as a liquid, that is generated by the weight of the medium itself) clearer. In other words: the pressure is generated because the medium is pressing against itself with its own weight.
One cmH2O corresponds roughly to one millibar, or, in SI units: 1,000 Pascal.
Hydrostatic pressure can be calculated using the following formula:
HSP = rho * g * h
, in which h (the height of the column of liquid), rho and g are physical constants.
Therefore, in the absence of other factors, the suction in an average adult can reach 50 cmH2O. The tapering of the ventricle produces this high amount of tensile stress due to the fact that the exterior surface of the brain is firmly attached to the skull and so, as a whole, cannot "shrink".
According to this physical formula, the effect will be reduced when a person is lying down, due to the fact that the height of the column will naturally be h = 0 cm, in turning meaning that HSP = 0. That is clearly very plausible, due to the fact that the column of liquid in a catheter that is "lying down" (horizontal) will no longer be squeezed out the end under the force of its own weight. In other words: the ventricle connected to the abdomen will no longer be at risk of being sucked dry either. In such a situation, there will no longer be any "siphoning effect".
It must again be emphasised that this physical effect will ALWAYS occur and is UNAVOIDABLE when the patient stands. When this happens, a huge suction force of 20 - 60 cmH2O will be produced, with the severity depending on the height of the patient or the distance between the ventricle and the diaphragm. The symptoms described above, such as slit ventricle syndrome and the movement of entire areas of the brain can be caused by this alone. Even this purely physical explanation makes it clear, that unlike underdrainage, the very similar symptoms caused by overdrainage ONLY occur when the patient is standing up, and will subside again relatively quickly once the patient lies down. This provides a good diagnostic criterion for when attempting to distinguish one of these phenomena from the other.
Still, low intraventricular pressure is not the primary issue when it comes to overdrainage. Rather, the primary issue is the fact that the expansion of the brain tissue can lead to the formation of hygromas and haematomata (i.e. cavities filled with liquid and blood) particularly when this occurs between the surface of the brain and the skull. The brain is firmly attached to the skull via the intricately-structured arachnoid mater and dura mater, which it usually cannot be detached from. If it is sucked, away from the cranial wall, with too much force these delicate membranes and the small bridging veins running through them will be the first things to rip and start bleeding. However, large venous sinuses in the dura or arteries can also be damaged. Overdrainage almost exclusively causes subdural haematomata (SDH). There is even the possibility that these may become chronic. Laboratory experiments have shown how a typical level of hydrostatic suction in the ventricles of around 30 cmH2O can subject the external membranes of the brain to forces equivalent to several hundred grams in weight. The scenario described above makes it clear how haematomata caused by overdrainage can become chronic (chronic subdural haematomata or cSDH).
Another equally serious problem induced by overdrainage is the so-called slit ventricle syndrome (SVS). Suction causes the ventricles to narrow, which can close the fine openings of the ventricular catheter (VC). The cerebrospinal fluid can then no longer be drained and the ventricles dilate again. If the suction then takes effect again, the process starts all over again. This periodic contraction and expansion of the ventricles, which is associated with strong tension, can already damage the tissue. If tissue then grows into the small catheter holes, this can lead to further tissue tears and bleeding during the subsequent ventricular expansion. If the ventricular catheter becomes irreversibly blocked, the ventricles remain narrow and shunt revision becomes unavoidable. As early as 1987, Rolf Gruber described this phase of SVS as follows:
"In shunt revision, the fixed ventricular catheter can only be removed with difficulty; its lumen is completely or partially obstructed by incarcerated tissue plaques in the ostia. They consist of vascularized neuroglia, plexus components and blood clots. Ependymal cells are only found on the torn tissue fragments outside, but never inside the catheter." [Gruber3, Das Schlitz-Ventrikel-Syndrom, 1987].
To this day, many authors assume that these demonstrable injuries to the ependyma due to chronic overdraining (i.e. not only in children) lead to a permanent stiffening/hardening of the ventricular walls and an associated reduction in brain compliance ("stiff ventricles"). This hypothesis is supported by the fact that the ventricles do not necessarily return to their original ventricular width once physiological, i.e. "normal" pressure conditions have been restored [Pollay4 1994, Ros5 2018, Wagner6 2018]. However, investigations into the contribution of pathological cell changes to this presumed ventricular stiffening have not yet been able to provide definitive clarity [Oi9 1986, Del Bigio7 2002, Di Rocco8 2015 ].
Lumbo-peritoneal (LP) shunts seem to have become increasingly popular in the last few years, as they make it possible to avoid skull and brain injuries. With LP shunts, the spinal cavity is directly linked to the ventricles. The Liquor is drained from between the third and fifth lumbar vertebrae into the peritoneum. Even today, the view that hydrostatic suction does not play a significant role in LP shunts and that the risk of overdrainage is therefore lower, can still be found in the literature (Bloch15, 2012). This assertion is utterly false and misleading. It probably comes from the fact that in LP shunts, the source of the Liquor (the spinal cavity) and drainage site (the peritoneum) are not located on top of one-another: the shunt catheter between the puncture site and abdomen is actually roughly horizontal, meaning that the hydrostatic level appears to be h = 0.

It has been explained previously, in detail, that when the person stands up, the entire Liquor filled spinal cavity, all the way up to the ventricles, is raised up above the abdomen. Indeed, if the cavity is now open because of the shunt, the column of liquid in the spine can drain into the abdomen, creating the same hydrostatic suction in the ventricles (the height, "h") as would occur in a ventriculo-peritoneal shunt. Given that the spinal cavity is significantly wider than the narrow peritoneal catheter and produces a greater flow of liquid, the overdrainage actually occurs significantly more quickly. In a recent literature review (Miyake16, 2016) a similar misleading remark is made, stating the siphon effect is negligible in LP shunts. However this should be viewed in a distinct way. It is true that in the case of the LP valve, which, of course, is at the same height as the abdomen, not very much suction comes from the abdomen (i.e. from below). Nevertheless, because of this, the entire force of the hydrostatic pressure in the spinal cavity weighs down upon it from above. In the case of a VP shunt, the valve is at the same height as the ventricle and all of the hydrostatic suction comes from below, whereas there is only a relatively low amount of IVP to exert an effect from above. Still, when it comes to a standard differential pressure valve, the only thing that is relevant is the sum of both the amount of pressure and the amount of suction. Therefore, in both cases, the outcome in relation to overdrainage remains the same.
Aside from the existing evidence and considerations of plausibility, it has not been possible to definitively establish whether a specific type of valve is better or worse, even in the last decade. In this area, scientific knowledge, which normally advances at high speed, appears to be progressing at a snail's pace. There are many very important reasons for this. One such reason is clearly the fact that human physiology is highly complex and that it often takes a huge amount of effort to collect reliable statistical data. As a result, it will be necessary to leave sufficient space in a later section for a nuanced weighing-up of the advantages and disadvantages posed by the different types of hydrostatic implants on the market.
Nevertheless, even at this stage, it can and should be pointed out that the effectiveness of gravitational valves in preventing overdrainage has been well-documented in the literature. This applies to every age group. The following list of recent studies only represents a sample of such literature: Al-Hakim17, 2018; Gebert18, 2016; Xinxing19, 2015, Suchorska20, 2015; Kehler21, 2015; Malem14, 2014; Thomale22, 2013; Lemcke23, 2013; Gebert24, 2013; Freimann25, 2013.
One finding of the SVASONA study (Lemcke23, 2013), which is well known amongst neurosurgeons, was that overdrainage can be prevented in one in three patients by using gravitational valves. The rate of overdrainage "in the control group" (i.e. the group who did not have any gravitational valves implanted) of this study was significantly higher than in other studies (Sundstrom26, 2017). However, the corresponding rate in the treatment group (who were implanted with gravitational valves) was also remarkably low. Alfred Aschoff, a hydrocephalus expert who is well known among neurosurgeons, has now noted, in his most-recently published textbook chapter on shunt valves, that this applies to many groups treated with gravitational valves (Aschoff27, 2019).
In this comprehensive text, Aschoff also superimposed the so-called "survival curves" (also known as “Kaplan-Meier curves") on top of one another, so that direct comparisons could be made. "Survival curves" are widely used in shunt therapy due to the fact that they provide a graphic representation (in percentage terms) of how many of the valves that were originally planted have "survived" (i.e. have continued to work) after a certain period of time. The valves not included in the survival curves were those subject to revision, i.e. those that were removed due to a malfunction or complication. Here too, the aforementioned problem of overdrainage is an important and sometimes irreparable complication, which can therefore shorten the service life of the valve. Other important reasons for shunt failure include infections, blockages and mechanical malfunctions, such as a valve breaking or a catheter being torn. In the survival curves compared by Aschoff, all the curves at the top are from studies using gravitational valves. Unlike standard differential valves, only 50-60% of which continued to operate without any malfunctions after a period of two years, around 80% of the gravitational valves were still in place following the same period of time.
The superiority of gravitational valves, as inferred by Aschoff, has even made its way into the official guidelines for the "Diagnostik und Therapie des Normaldruckhydrozephalus" [Diagnosis and Treatment of Normal Pressure Hydrocephalus], which is published periodically by the German Neurological society:
www.awmf.org/uploads/tx_szleitlinien/030-063_S1_Normaldruckhydrozephalus_2018-03.pdf
It is stated, under the heading "Important Recommendations", that,
"If a shunt is implanted to treat idiopathic normal pressure hydrocephalus, a gravitational valve should be used. Differential pressure valves, especially those with in which the valve opening pressure cannot be adjusted, lead to complications relating to overdrainage significantly more often under the same clinical conditions".
Bibliography
- Ritz R, Roser F, Morgalla M, Dietz K, Tatagiba M, Will BE. Do antibiotic-impregnated shunts in hydrocephalus therapy reduce the risk of infection? An observational study in 258 patients. BMC Infect Dis. 2007 May 8;7:38. doi: 10.1186/1471-2334-7-38.
- Parker SL, McGirt MJ, Murphy JA, Megerian JT, Stout M, Engelhart L. Comparative effectiveness of antibiotic-impregnated shunt catheters in the treatment of adult and pediatric hydrocephalus: analysis of 12,589 consecutive cases from 287 US hospital systems. J Neurosurg. 2015 Feb;122(2):443-8. doi: 10.3171/2014.10.JNS13395. Epub 2014 Nov 21.
- Gruber R. Das Schlitzventrikel-Syndrom: die hydrostasebedingte Shuntkomplikationen des kindlichen Hydrozephalus. In: Bibliothek für Kinderchirurgie. Stuttgart: Hippokrates Verlag; 1987. Kapitel 9.
- Pollay M. Slit ventricle syndrome. Crit Rev Neurosurg. 1994;4:51-56.
- Ros B, Iglesias S, Martín Á, Carrasco A, Ibáñez G, Arráez MA. Shunt overdrainage syndrome: review of the literature. Neurosurg Rev. 2018 Oct;41(4):969-981. doi: 10.1007/s10143-017-0849-5. Epub 2017 Mar 29.
- Wagner W. Therapie des Hydrozephalus. In: Pädiatrische Neurochirurgie. 1. Aufl. Springer Verlag; 2018. p. 261-271.
- Del Bigio MR. Neuropathological findings in a child with slit ventricle syndrome. Pediatr Neurosurg. 2002 Sep;37(3):148-151. doi: 10.1159/000064396
- Di Rocco C, Turgut M, Jallo G, Martinez-Lage JF. Cranio-cerebral disproportion as a late complication. In: Di Rocco C, Turgut M, Jallo G, Martinez-Lage JF, editors. Complications of CSF Shunting in Hydrocephalus: Prevention, Identification and Management. Berlin: Springer; 2015. p. 233-246.
- Oi S, Matsumoto S. Morphological findings of postshunt slit-ventricle in experimental canine hydrocephalus. Aspects of causative factors of isolated ventricles and slit-ventricle syndrome. Childs Nerv Syst. 1986;2(4):179-84. doi: 10.1007/BF00706807.
- Bergsneider M, Yang I, Hu X, McArthur DL, Cook SW, Boscardin WJ. Relationship between valve opening pressure, body position, and intracranial pressure in normal pressure hydrocephalus: paradigm for selection of programmable valve pressure setting. Neurosurgery. 2004 Oct;55(4):851-8; discussion 858-9. doi: 10.1227/01.neu.0000137631.42164.b8.
- Pedersen SH, Lilja-Cyron A, Andresen M, Juhler M. The Relationship Between Intracranial Pressure and Age-Chasing Age-Related Reference Values. World Neurosurg. 2018 Feb;110:e119-e123. doi: 10.1016/j.wneu.2017.10.086. Epub 2017 Oct 26.
- Antes S, Stadie A, Müller S, Linsler S, Breuskin D, Oertel J. Intracranial Pressure-Guided Shunt Valve Adjustments with the Miethke Sensor Reservoir. World Neurosurg. 2018 Jan;109:e642-e650. doi: 10.1016/j.wneu.2017.10.044. Epub 2017 Oct 17.
- Boon AJ, Tans JT, Delwel EJ, Egeler-Peerdeman SM, Hanlo PW, Wurzer HA, Avezaat CJ, de Jong DA, Gooskens RH, Hermans J. Dutch Normal-Pressure Hydrocephalus Study: randomized comparison of low- and medium-pressure shunts. J Neurosurg. 1998 Mar;88(3):490-5. doi: 10.3171/jns.1998.88.3.0490.
- Malem DN, Shand Smith JD, Toma AK, Sethi H, Kitchen ND, Watkins LD. An investigation into the clinical impacts of lowering shunt opening pressure in idiopathic normal pressure hydrocephalus: A case series. Br J Neurosurg. 2015 Feb;29(1):18-22. doi: 10.3109/02688697.2014.950630. Epub 2014 Aug 21.
- Bloch O, McDermott MW. Lumboperitoneal shunts for the treatment of normal pressure hydrocephalus. J Clin Neurosci. 2012 Aug;19(8):1107-1111. doi: 10.1016/j.jocn.2011.11.019.
- Miyake H. Shunt Devices for the Treatment of Adult Hydrocephalus: Recent Progress and Characteristics. Neurol Med Chir (Tokyo). 2016 May 15;56(5):274-83. doi: 10.2176/nmc.ra.2015-0282. Epub 2016 Apr 4.
- Al-Hakim S, Schaumann A, Schneider J, Schulz M, Thomale UW. Experience in shunt management on revision-free survival in infants with myelomeningocele. Childs Nerv Syst. 2018 Jul;34(7):1375-1382. doi: 10.1007/s00381-003-0759-4.
- Gebert AF, Schulz M, Schwarz K, Thomale UW. Long-term survival rates of gravity-assisted, adjustable differential pressure valves in infants with hydrocephalus. J Neurosurg Pediatr. 2016 May;17(5):544-551.
- Xinxing L, Hongyu D, Yunhui L. Using individualized opening pressure to determine the optimal setting of an adjustable proGAV shunt in treatment of hydrocephalus in infants. Childs Nerv Syst. 2015 Aug;31(8):1267-1271.
- Suchorska B, Kunz M, Schniepp R, Jahn K, Goetz C, Tonn JC, Peraud A. Optimized surgical treatment for normal pressure hydrocephalus: comparison between gravitational and differential pressure valves. Acta Neurochir (Wien). 2015 Apr;157(4):703-9. doi: 10.1007/s00701-015-2345-4. Epub 2015 Feb 11.
- Kehler U, Kiefer M, Eymann R, Wagner W, Tschan CA, Langer N, Rohde V, Ludwig HC, Gliemroth J, Meier U, Lemcke J, Thomale UW, Fritsch M, Krauss JK, Mirzayan MJ, Schuhmann M, Huthmann A. PROSAIKA: a prospective multicenter registry with the first programmable gravitational device for hydrocephalus shunting. Clin Neurol Neurosurg. 2015 Oct;137:132-136.
- Thomale UW, Gebert AF, Haberl H, Schulz M. Shunt survival rates by using the adjustable differential pressure valve combined with a gravitational unit (proGAV) in pediatric neurosurgery. Childs Nerv Syst. 2013 Mar;29(3):425-31. doi: 10.1007/s00381-012-1956-9. Epub 2012 Nov 8.
- Lemcke J, Meier U, Müller C, Fritsch MJ, Kehler U, Langer N, Kiefer M, Eymann R, Schuhmann MU, Speil A, Weber F, Remenez V, Rohde V, Ludwig HC, Stengel D. Safety and efficacy of gravitational shunt valves in patients with idiopathic normal pressure hydrocephalus: a pragmatic, randomised, open label, multicentre trial (SVASONA). J Neurol Neurosurg Psychiatry. 2013 Aug;84(8):850-7. doi: 10.1136/jnnp-2012-303936. Epub 2013 Mar 1.
- Gebert AF, Schulz M, Haberl H, Thomale UW. Adjustments in gravitational valves for the treatment of childhood hydrocephalus: a retrospective survey. Childs Nerv Syst. 2013 Nov;29(11):2019-2025. doi: 10.1007/s00381-021-05250-4.
- Freimann FB, Vajkoczy P, Sprung C. Patients benefit from low-pressure settings enabled by gravitational valves in normal pressure hydrocephalus. Clin Neurol Neurosurg. 2013 Oct;115(10):1982-6. doi: 10.1016/j.clineuro.2013.06.010. Epub 2013 Jul 4.
- Sundström N, Lagebrant M, Eklund A, Koskinen LD, Malm J. Subdural hematomas in 1846 patients with shunted idiopathic normal pressure hydrocephalus: treatment and long-term survival. J Neurosurg. 2018 Sep;129(3):797-804. doi: 10.3171/2017.5.JNS17481. Epub 2017 Oct 27.
- Aschoff A. In-depth view: functional characteristics of CSF shunt devices (pros and cons). In: Di Rocco C, editor. Textbook of Pediatric Neurosurgery. Cham: Springer; 2019.