“A shunt was considered infected if the patient showed clinical signs of wound infection, septicemia in patients with ventriculoatrial shunts, peritonitis in patients with ventriculoperitonealshunts, or meningitis and if bacteria were cultivated from blood, peritoneum, cerebrospinal fluid, or the shunt system. An acute infection was an infection causing symptoms and/or positive bacteriological cultures within the first four postoperative weeks. If the same criteria were fulfilled more than one month postoperatively it was registered as a late infection.”
Some typical and common symptoms are fever, pain and also redness and swelling of the affected areas of the body, as far as local infections are concerned. However, it must be emphasized here that a shunt infection is by no means always easy and clear to diagnose. As part of the large current BASICS study from 2019, which will be discussed several times in this article, the existence or non-existence of an infection was determined by a (blinded) expert panel (“central primary outcome review panel”) on the basis of a comprehensive list of criteria described by Mallucci et al. [5; 6].
“These infections (of a cerebrospinalfluid shunt) may be difficult to diagnose because changes in cerebrospinal fluid parameters are often subtle, making it hard to determine if the abnormalities are related to infection, related to placement of devices, or following neurosurgery.”
While they are often hard to diagnose with certainty, shunt infections still are one of the most common complications of shunt surgery. In a comprehensive shunt therapy database analysis from two large German hospitals by Bock et al. [8] from 2018, the infection risk ranked fourth as a cause for revision, behind catheter migration, obstruction and unresolved causes. This evaluation is based on 256 pediatric cases followed up for a median of 8.5 years. What stands out in this analysis is that revisions (i.e. explantations of the shunt) due to infections occur predominantly in the first 12 months. In another evaluation of the large “UK-Shunt-Registry” data, encompassing 41,036 procedures in 26,545 patients, Fernández-Méndez et al. [9] found that shunt infection ranked second in reasons for primary shunt revisions during their ten-year study period. ("Primary" here means that only shunts that were the patients' first were considered).
The same conclusion was reached in the particularly comprehensive meta-analysis by Isaacs et al. from 2023, which was based on 38,095 shunt implantations in adults [2]: Infections are the second most common complication after obstructions and are causative for 22.5% of all revisions.
As infections appear to be one of the most common and serious complications since the introduction of shunt therapy, there are a large number of other studies documenting infection rates depending on various factors. Some typical influencing factors are: different patient age groups and etiologies, number of shunt-implantations/revisions per patient, varying hospital infrastructures, surgeon expertise, applied protocols and protocol compliance. Accordingly, these figures show an enormous range. In a very early long-term study by George et al. this wide range and its development from 1952 till 1976 (25 years) is impressively illustrated [11].
This early study is based on 840 operations ("procedures") in 410 patients of all ages. However, the most important facts concerning shunt infections in general were already noted in this publication:
- In the early days of shunt therapy in the 1950s, infection rates above 35% were possible.
- Since then, the infection rate has decreased significantly and continuously.
- Especially the mortality from infection dropped from 35% to 6% (in 1976).
- The vast majority of infections are so-called "early-infections", which occur within the first month after implantation, in this case two-thirds of all infections [11].
- Higher infection rates occur in very young patients (< 1 year), in whom the immune system is still weak.
- In “follow-up shunts”, i.e. non-primary shunts, comparatively higher infection rates occur.
- A 25-fold variance in infection rates was found between different surgeons and could be attributed, amongst others, to their individual experience and technique
- More than 60% of infections are caused by the pathogen Staphylococcus, predominantly Staphylococcus epidermis, but also Staphylococcus aureus.
These basic facts have been confirmed again and again in subsequent studies:
- The dominance of "early infections" (as opposed to "late infections") has been confirmed by several studies [4; 8; 10]: Of particular note is the 2017 analysis of data from the "UK shunt registry" by Pickard et al [10] (see www.sbns.org.uk/index.php/audit/shunt-registry/ ). Based on a large data set of 53,767 operations in 29,341 patients, the time course of the occurrence of the various possible shunt complications over 6 years was recorded and compared (see Figure 4). This studies clearly shows that the vast majority of infections occur very early (in the first 4 weeks).

- The increased vulnerability of children and newborns, especially premature infants has also been repeatedly verified later on [12; 13; 14; 15; 16].
- Finally, also the assumption, that surgeon experience has a significant impact on infection rates is expressed several times. The helpful influence of the experienced neurosurgeon could be effective either by shortening the operating time, but possibly also by changing the behavior of all those involved in complying with the hygiene protocol [4; 13; 14; 16; 17; 18].
In addition to the points listed above, the following risk factors are repeatedly mentioned in the larger studies and reviews [12; 13; 15; 16; 19; 20; 21]:
- previous shunt revisions
- certain etiologies of hydrocephalus, especially after previous infections and intraventricular hemorrhage (IVH= intraventricular hemorrhage), myelomeningocele, children with malignant disease, in case of chemotherapy-associated immunosuppression, after long-term application of steroids above the Cushing threshold
- patients who experienced post-operative CSF leaks, as they provide an entry way for bacteria
An interesting result of a large worldwide ISPN (International Society For Pediatric Neurosurgery) survey on neurosurgical practices regarding the management of shunt infections from 2020 should also be mentioned here: This survey showed a significantly lower risk of shunt infection in hospitals with particularly high pediatric-focused case volumes [22]. The background to this is not yet understood. However, it is possible that the higher level of clinical experience in such hospitals mentioned above plays a decisive role here.
Infection rates today
Since the 2020s, a range between 5% and 17% for possible infection rates is specified by several authors [5; 9; 23].
In the large prospective BASICS study [5] (see chapter 3) a mean infection rate of 6% has been found in 536 patients of mixed age and etiology, using standard-catheters, but only for primary shunts. In this study, any additional, special infection-prevention measures taken were randomly distributed (because it was entirely the responsibility of the surgeon), so that the 6% quoted actually represents a "mean value" for primary shunts over all age-groups.
In the aforementioned 2020 ISPN global survey, Behbahani et al. report that 64% of the respondents declared infection rates of less than 5% while 22% of the practitioners mentioned infection rates of 5-10% in the pediatric patients.
Similarly, in their study based on 4,913 procedures performed in 13 hospitals of the “Hydrocephalus Clinical Research Network” (HCRN), Chu et al. found an average infection rate of 5.1% in children, with rates ranging from 2.2% to 9.3% per center [24]. And finally, based on 558 procedures, Shibamura-Fujiogi et. al. report similar values, i.e. 4.3%. [25].
This overall drop in infection rates – compared to early numbers – demonstrates how awareness for risk factors and implementation of measures aimed at their reduction have already been successful. At the same time, the high variance between centers shows that a standardized, evidence-based approach could benefit patients and healthcare systems.
How a shunt gets infected
Understanding the causes and progression of shunt infections is essential in order to effectively address them. From the dominance of early infections within the first 4 weeks, it seems obvious that at least this type of infection occurs intra-operatively [16; 26]. This assumption is further supported by the type of the predominantly found germs in early infections, which often are "skin derived" bacteria. These bacteria are found in normal skin flora, such as coagulase-negative Staphylococci (CoNS), e.g. Staphylococcus epidermidis and Staphylococcus aureus [4; 14; 26]. CoNS account for 50% and Staphylococcus aureus for 33% of all shunt infections [26].

The extensive data from the BASICS study was also analyzed with regard to the causative germs, which led to similar results as Wells et al. In addition, the pathogens found in this evaluation were classified on the basis of Gram staining, as this is decisive for the choice of possible antibiotics [5; 6] (see also the “Nerdbox”):
- gram-positive bacteria make up around 73% of overall shunt infections
- gram-negative bacteria make up around 20% of overall shunt infections
- mixed bacteria make up around 7% of overall shunt infections
Contamination of the implant occurs either through direct contact with the patient's skin or that of one of the people present. After the occupation of the implant, the pathogens rapidly adhere to the biomaterial surface and deploy strategies to escape both the immune system and potential antibiotic treatment [27]. Skin commensals like Staphylococci attach to the silicone surface of the shunt tubing, where they start to proliferate. Due to the hostile environment in CSF, with low iron contents and insufficient carbon and nitrogen sources, this proliferation is slow but steady [14]. Grampositive bacteria like CoNS and Staphylococcus aureus usually occur in clusters and show a propensity for the formation of so-called biofilms, allowing them to effectively attach to implant materials. Interestingly, the first report of such a biofilm in 1972 in a medical device was from a shunt infection [28].
The development of such a biofilm protects their further growth and makes antibiotic treatment much more difficult. In general, biofilms are groups of microorganisms attached to biotic or abiotic surfaces and surrounded by a matrix composed of an extracellular polymeric substance (EPS), which includes extracellular DNA, proteins and polysaccharide. These biofilms act as physical barriers against the body´s immune response as well as drugs [29]. What is more, when bacteria attach to the catheter surface and develop a biofilm, they change their metabolism in order to conserve energy. This state is called “dormancy” and reduces many of the metabolic target sites for common antibiotics, making them less effective [30; 31]. Reduced permeability of the biofilm and bacterial dormancy mean that much higher – often unacceptably high – concentrations of antibiotics are needed to effectively fight the bacteria [14].
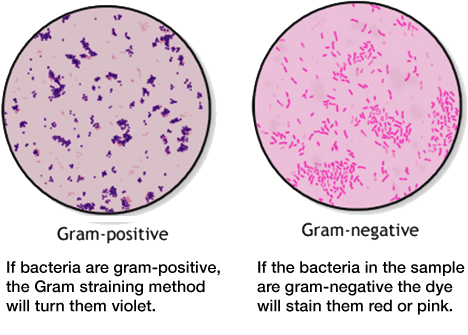
gram positive vs gram-negative bacteria
Infections can be caused by different agents such as fungi, parasites, algea, viruses, prions or bacteria. Bacteria can be further classified by shape: spherical (cocci), rod (bacilli), spiral (spirilla), comma (vibrios) or corkscrew (spirochaetes). They can exist as single cells, in pairs, chains or clusters.
Another method of differentiation is the so-called Gram staining method, named after the Danish bacteriologist Hans Christian Gram. It is one of the most important staining techniques in microbiology and it is usually the first test performed for the identification of bacteria. The Gram stain differentiates bacteria into Gram-negative and Gram-positive based on the chemical and physical properties of their cell walls. The methods consists of a series of staining and decolorization steps. Gram-negative cells will stain red to pink, Gram-positive cells will stain blue to purple.
(https://www.cdc.gov/labtraining/docs/job_aids/routine_microscopy_procedures/Gram-Stain_508.pdf)
The difference in Gram stainability is due to the different structure of the bacteria's cell walls. Gram staining is important in the diagnosis and especially the treatment of infectious diseases. Gram-positive and Gram-negative bacteria can often only be combated with different antibiotics.