Shunt infection: How it develops and how it can be prevented
Preface
To date, the implantation of a shunt has become the most common treatment of hydrocephalus [2; 3]. While considered a relatively easy and routine procedure, nonetheless the implantation of a shunt system requires the introduction of foreign materials into the body: catheters, valves and optional components like reservoirs are implanted “subcutaneously” (i.e. under the skin). Accordingly, hydrocephalus shunt therapy is (also) unavoidably associated with a risk of infection.
show moreThis article deals in detail with the frequent and serious “side effect” of infection in case of hydrocephalus shunt implantation, i.e. with its importance for the patient and the healthcare system, its origin and development, its effects and the current possibilities for prevention. The article also serves to provide a selected overview of the vast amount of literature available on this topic and to present the current state of knowledge, as far as possible. In particular, we tried to prefer comprehensive studies with large case numbers and so called metareviews or guidelines, where available. We also tried to compare older and current studies, to show the development over time.

“A shunt was considered infected if the patient showed clinical signs of wound infection, septicemia in patients with ventriculoatrial shunts, peritonitis in patients with ventriculoperitonealshunts, or meningitis and if bacteria were cultivated from blood, peritoneum, cerebrospinal fluid, or the shunt system. An acute infection was an infection causing symptoms and/or positive bacteriological cultures within the first four postoperative weeks. If the same criteria were fulfilled more than one month postoperatively it was registered as a late infection.”
Some typical and common symptoms are fever, pain and also redness and swelling of the affected areas of the body, as far as local infections are concerned. However, it must be emphasized here that a shunt infection is by no means always easy and clear to diagnose. As part of the large current BASICS study from 2019, which will be discussed several times in this article, the existence or non-existence of an infection was determined by a (blinded) expert panel (“central primary outcome review panel”) on the basis of a comprehensive list of criteria described by Mallucci et al. [5; 6].
“These infections (of a cerebrospinalfluid shunt) may be difficult to diagnose because changes in cerebrospinal fluid parameters are often subtle, making it hard to determine if the abnormalities are related to infection, related to placement of devices, or following neurosurgery.”
While they are often hard to diagnose with certainty, shunt infections still are one of the most common complications of shunt surgery. In a comprehensive shunt therapy database analysis from two large German hospitals by Bock et al. [8] from 2018, the infection risk ranked fourth as a cause for revision, behind catheter migration, obstruction and unresolved causes. This evaluation is based on 256 pediatric cases followed up for a median of 8.5 years. What stands out in this analysis is that revisions (i.e. explantations of the shunt) due to infections occur predominantly in the first 12 months. In another evaluation of the large “UK-Shunt-Registry” data, encompassing 41,036 procedures in 26,545 patients, Fernández-Méndez et al. [9] found that shunt infection ranked second in reasons for primary shunt revisions during their ten-year study period. ("Primary" here means that only shunts that were the patients' first were considered).
The same conclusion was reached in the particularly comprehensive meta-analysis by Isaacs et al. from 2023, which was based on 38,095 shunt implantations in adults [2]: Infections are the second most common complication after obstructions and are causative for 22.5% of all revisions.
As infections appear to be one of the most common and serious complications since the introduction of shunt therapy, there are a large number of other studies documenting infection rates depending on various factors. Some typical influencing factors are: different patient age groups and etiologies, number of shunt-implantations/revisions per patient, varying hospital infrastructures, surgeon expertise, applied protocols and protocol compliance. Accordingly, these figures show an enormous range. In a very early long-term study by George et al. this wide range and its development from 1952 till 1976 (25 years) is impressively illustrated [11].
This early study is based on 840 operations ("procedures") in 410 patients of all ages. However, the most important facts concerning shunt infections in general were already noted in this publication:
- In the early days of shunt therapy in the 1950s, infection rates above 35% were possible.
- Since then, the infection rate has decreased significantly and continuously.
- Especially the mortality from infection dropped from 35% to 6% (in 1976).
- The vast majority of infections are so-called "early-infections", which occur within the first month after implantation, in this case two-thirds of all infections [11].
- Higher infection rates occur in very young patients (< 1 year), in whom the immune system is still weak.
- In “follow-up shunts”, i.e. non-primary shunts, comparatively higher infection rates occur.
- A 25-fold variance in infection rates was found between different surgeons and could be attributed, amongst others, to their individual experience and technique
- More than 60% of infections are caused by the pathogen Staphylococcus, predominantly Staphylococcus epidermis, but also Staphylococcus aureus.
These basic facts have been confirmed again and again in subsequent studies:
- The dominance of "early infections" (as opposed to "late infections") has been confirmed by several studies [4; 8; 10]: Of particular note is the 2017 analysis of data from the "UK shunt registry" by Pickard et al [10] (see www.sbns.org.uk/index.php/audit/shunt-registry/ ). Based on a large data set of 53,767 operations in 29,341 patients, the time course of the occurrence of the various possible shunt complications over 6 years was recorded and compared (see Figure 4). This studies clearly shows that the vast majority of infections occur very early (in the first 4 weeks).

- The increased vulnerability of children and newborns, especially premature infants has also been repeatedly verified later on [12; 13; 14; 15; 16].
- Finally, also the assumption, that surgeon experience has a significant impact on infection rates is expressed several times. The helpful influence of the experienced neurosurgeon could be effective either by shortening the operating time, but possibly also by changing the behavior of all those involved in complying with the hygiene protocol [4; 13; 14; 16; 17; 18].
In addition to the points listed above, the following risk factors are repeatedly mentioned in the larger studies and reviews [12; 13; 15; 16; 19; 20; 21]:
- previous shunt revisions
- certain etiologies of hydrocephalus, especially after previous infections and intraventricular hemorrhage (IVH= intraventricular hemorrhage), myelomeningocele, children with malignant disease, in case of chemotherapy-associated immunosuppression, after long-term application of steroids above the Cushing threshold
- patients who experienced post-operative CSF leaks, as they provide an entry way for bacteria
An interesting result of a large worldwide ISPN (International Society For Pediatric Neurosurgery) survey on neurosurgical practices regarding the management of shunt infections from 2020 should also be mentioned here: This survey showed a significantly lower risk of shunt infection in hospitals with particularly high pediatric-focused case volumes [22]. The background to this is not yet understood. However, it is possible that the higher level of clinical experience in such hospitals mentioned above plays a decisive role here.
Infection rates today
Since the 2020s, a range between 5% and 17% for possible infection rates is specified by several authors [5; 9; 23].
In the large prospective BASICS study [5] (see chapter 3) a mean infection rate of 6% has been found in 536 patients of mixed age and etiology, using standard-catheters, but only for primary shunts. In this study, any additional, special infection-prevention measures taken were randomly distributed (because it was entirely the responsibility of the surgeon), so that the 6% quoted actually represents a "mean value" for primary shunts over all age-groups.
In the aforementioned 2020 ISPN global survey, Behbahani et al. report that 64% of the respondents declared infection rates of less than 5% while 22% of the practitioners mentioned infection rates of 5-10% in the pediatric patients.
Similarly, in their study based on 4,913 procedures performed in 13 hospitals of the “Hydrocephalus Clinical Research Network” (HCRN), Chu et al. found an average infection rate of 5.1% in children, with rates ranging from 2.2% to 9.3% per center [24]. And finally, based on 558 procedures, Shibamura-Fujiogi et. al. report similar values, i.e. 4.3%. [25].
This overall drop in infection rates – compared to early numbers – demonstrates how awareness for risk factors and implementation of measures aimed at their reduction have already been successful. At the same time, the high variance between centers shows that a standardized, evidence-based approach could benefit patients and healthcare systems.
How a shunt gets infected
Understanding the causes and progression of shunt infections is essential in order to effectively address them. From the dominance of early infections within the first 4 weeks, it seems obvious that at least this type of infection occurs intra-operatively [16; 26]. This assumption is further supported by the type of the predominantly found germs in early infections, which often are "skin derived" bacteria. These bacteria are found in normal skin flora, such as coagulase-negative Staphylococci (CoNS), e.g. Staphylococcus epidermidis and Staphylococcus aureus [4; 14; 26]. CoNS account for 50% and Staphylococcus aureus for 33% of all shunt infections [26].

The extensive data from the BASICS study was also analyzed with regard to the causative germs, which led to similar results as Wells et al. In addition, the pathogens found in this evaluation were classified on the basis of Gram staining, as this is decisive for the choice of possible antibiotics [5; 6] (see also the “Nerdbox”):
- gram-positive bacteria make up around 73% of overall shunt infections
- gram-negative bacteria make up around 20% of overall shunt infections
- mixed bacteria make up around 7% of overall shunt infections
Contamination of the implant occurs either through direct contact with the patient's skin or that of one of the people present. After the occupation of the implant, the pathogens rapidly adhere to the biomaterial surface and deploy strategies to escape both the immune system and potential antibiotic treatment [27]. Skin commensals like Staphylococci attach to the silicone surface of the shunt tubing, where they start to proliferate. Due to the hostile environment in CSF, with low iron contents and insufficient carbon and nitrogen sources, this proliferation is slow but steady [14]. Grampositive bacteria like CoNS and Staphylococcus aureus usually occur in clusters and show a propensity for the formation of so-called biofilms, allowing them to effectively attach to implant materials. Interestingly, the first report of such a biofilm in 1972 in a medical device was from a shunt infection [28].
The development of such a biofilm protects their further growth and makes antibiotic treatment much more difficult. In general, biofilms are groups of microorganisms attached to biotic or abiotic surfaces and surrounded by a matrix composed of an extracellular polymeric substance (EPS), which includes extracellular DNA, proteins and polysaccharide. These biofilms act as physical barriers against the body´s immune response as well as drugs [29]. What is more, when bacteria attach to the catheter surface and develop a biofilm, they change their metabolism in order to conserve energy. This state is called “dormancy” and reduces many of the metabolic target sites for common antibiotics, making them less effective [30; 31]. Reduced permeability of the biofilm and bacterial dormancy mean that much higher – often unacceptably high – concentrations of antibiotics are needed to effectively fight the bacteria [14].
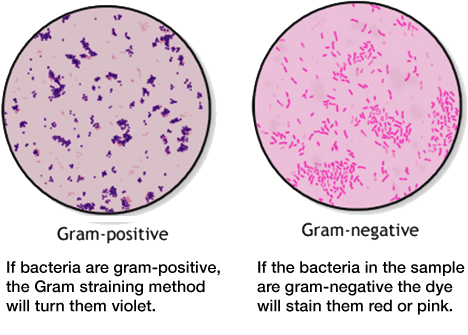
gram positive vs gram-negative bacteria
Infections can be caused by different agents such as fungi, parasites, algea, viruses, prions or bacteria. Bacteria can be further classified by shape: spherical (cocci), rod (bacilli), spiral (spirilla), comma (vibrios) or corkscrew (spirochaetes). They can exist as single cells, in pairs, chains or clusters.
Another method of differentiation is the so-called Gram staining method, named after the Danish bacteriologist Hans Christian Gram. It is one of the most important staining techniques in microbiology and it is usually the first test performed for the identification of bacteria. The Gram stain differentiates bacteria into Gram-negative and Gram-positive based on the chemical and physical properties of their cell walls. The methods consists of a series of staining and decolorization steps. Gram-negative cells will stain red to pink, Gram-positive cells will stain blue to purple.
(https://www.cdc.gov/labtraining/docs/job_aids/routine_microscopy_procedures/Gram-Stain_508.pdf)
The difference in Gram stainability is due to the different structure of the bacteria's cell walls. Gram staining is important in the diagnosis and especially the treatment of infectious diseases. Gram-positive and Gram-negative bacteria can often only be combated with different antibiotics.

How to treat shunt infections
Once the infection has manifested itself, thus, treatment faces many difficulties: Many antibiotics given intravenously fail to achieve sufficient concentration in the CSF, meaning that antibiotic levels remain below the level needed to effectively fight the bacteria, who, moreover, are protected by their dormant state in the biofilm [14]. Intravenous or oral antibiotic treatment alone is therefore mostly insufficient. Only selected, early shunt infections can be treated with special biofilm-active antibiotics [32]. In most cases, thus, the treatment of choice is the removal of the infected shunt, systemic antibiotic treatment, placement of an external drain if necessary, followed by the implantation of a new shunt after the infection has cleared off [4; 14; 33].
For the patient, this treatment requires at least two additional surgeries as well as a prolonged hospital stay together with a burdensome prolonged antibiosis. Every infection is a heavy burden for each patientEspecially in children, such infection episodes are also often associated with impaired cognitive function and subsequent impaired school performance [16]. Repeat surgeries and prolonged hospital stays, moreover, also lead to significantly higher costs for the healthcare system [5; 6; 34]. Therefore, it is of central importance to prevent infection from the beginning and take appropriate prophylactical measures.
Prevention is better than treatment
Effective prophylaxis can only consist of eliminating existing germs before the operation and preventing the transmission of new germs during the operation as far as possible. Specifically, the following measures are used in practice:
a) Prevention or reduction of the penetration of germs by introducing and consistently adhering to strict hygiene protocols, before, during and after the operation
b) local (also over a wide area of the body) application of antiseptic disinfectants, such as povidone iodine or chlorhexidine
c) prophylactic systemic and/or local administration of antibiotics, before, during and after the operation
It is important to emphasize that these measures are not mutually exclusive, but complementary. Many studies suggest combining organizational AND hygienic measures to avoid infection risks [13; 15; 17; 36; 37; 38]. The focus here is on patient and staff preparation, OR management, special surgical techniques and the additional local and systemic use of disinfectants and antibiotics. Usually, these measures are summarized in "protocols", some of which are explained in the following section.
Measures of a possible hygiene protocol
One of the first published protocols of this kind is that of Choux et al. from 1992 [17]. Elements included in their protocol were:
- early morning scheduling - before other operations
- operation of neonates and infants before older children
- limitation of shunt procedures per day
- limited time of operation (20 to 40 mins)
- limited traffic and staff present in operating room
- experienced neurosurgeon performing the operation
- opening sterile packing at last moment
- shunt materials rinsed with antibiotics and intravenous antibiotics before skin incisions.
After introduction of the protocol between 1983 and 1990, incidence on shunt infection was dramatically reduced from approx. 15% to 0.33% per patient and from approx. 8% to 0.17% per procedure [17].
Later on, this protocol was adapted by different neurosurgical units, so that a variety of single measures have been tested today. For pediatric patients, the detailed protocols published by the “Hydrocephalus Clinical Research Network” (HCRN) in USA are a noteworthy example. The HCRN is a huge collaboration of pediatric neurosurgical centers conducting systematic investigations in the management of pediatric hydrocephalus. Based on one of their findings, namely that the lack of proper hand-washing technique by the whole surgical team was an important risk factor for infection, they included a formal surgical scrub with povidone iodine or chlorhexidine scrub brushes, not just use of an antiseptic cream [37]. With introduction of this protocol, published in 2011, the infection rates could be reduced from 8.8% prior to the protocol to 5.7%.
Okamura et al. [23] published a protocol for an older patient group in 2020, which allowed them to reduce shunts infections from 7.3% to 0%. The average age of the patients before and after protocol implementation was around 59 years. Their protocol includes:
- Scheduling of shunt surgeries as the first procedure in each operating room
- minimization of door opening and number of surgical staff members
- double gloving with change of external gloves just before skin incision
- opening of the sterile packaging with the implant just before the skin incision and immersion in vancomycin solution
- injection of vancomycin and gentamycin into the reservoir after implantation
- postoperative management of oxygenation and strict glycemic control
- postoperative intravenous antibiotic drip
- dressing change at least once within 24 hours
The presented protocols nicely demonstrate that there is a lot of overlap between the measures different centers take to reduce shunt infections. Overall, there are many possible individual measures, whose evaluation has in some cases changed over time.
For example, Faillache developed the "no-touch protocol" already in 1995 and demonstrated its effectiveness, at least in his own study. Within this method, the implants are only touched and inserted with sterile instruments as far as possible [13; 15; 37; 39].
Today, the preoperative hair shaving over the incision site also mentioned in the “no-touch protocol” is no longer considered necessary or effective [14]. Instead, clinics or surgeons can opt for a no-shave policy. Some protocols combine this with a preoperative chlorhexidine hair wash and shower or bath [13; 15; 37].
The other way round, the practice of “double-gloving”, which was also already documented by Faillace [39], is generally accepted today as a good measure to minimize contamination risks. Gloves can become contaminated early in the procedure through contact with skin. “Double gloving” means that the outer pair can be removed or changed prior to device manipulation [15; 37; 40; 41].
Some other protocols also tested antimicrobial sutures and drapes [15; 37; 42]. In a small, randomized controlled trial, Rozelle et al. tested the effect of triclosan-coated sutures. Use of these sutures led to a reduction of infections from 21% in the control group to 4.3% in the group with triclosan-coated sutures, suggesting that some infections resulted from postoperative wound suppurations [14; 42]. Iodine-impregnated incision drapes can be useful to cover the cloth drapes and provide a dry aseptic surface [13; 15; 43].
Of course, each single measure in a protocol can only have a prophylactic effect, if it is strictly adhered to and if deviations are detected and remedied. Among others, Hommelstad and Bayston pointed out the crucial importance of protocol compliance for their success [13; 14]. Especially when many different people participate in the surgery, such as students in academic teaching hospitals, the presence of an experienced neurosurgeon might be helpful and might have an impact not only on the on the duration of the operation, but also on the behavior of all participants, meaning a stricter compliance to the protocol.
Systemic vs. local/topical prophylactic antibiosis
The protocols discussed so far have also shown that the peri-operative use of intravenously administered antibiotics (“systemic antibiosis”) is a frequently used measure. This appears problematic, especially considering the timely penetration of intravenous antibiotics into the CSF. Despite using such antibiotic prophylaxis, several centers reported rates considered unacceptably high [12; 14; 15; 44]. The use of prophylactic antibiosis is related to the question of the advantages and disadvantages of systemic or local antibiotic prophylaxis, which are often combined to combat surgical implant infections.
As discussed above, due to the metabolic changes of the bacteria in the biofilm very high antibiotic concentrations are needed to neutralize them. Furthermore, the CSF penetration of some antibiotics is limited [14; 32]. Systemic use, thus, often fails to achieve sufficiently high concentrations in the CSF and therefore might not have the desired impact neither in the treatment, nor in the prevention of infections. In contrast, local or topical applications of antimicrobial agents can deliver higher drug levels directly on the implant surface and in its vicinity. Their aim is to prevent organisms from colonizing the implant in the first place and to protect the tissue around the implant [1].
Evidence for the infection prevention protocol elements
As discussed above, there are many different prophylactic measures, most of which seems immediately plausible and are also used in practice. But the “level of clinical evidence” for the different measures varies, with most of them lacking a high level of evidence. This refers to reliable clinical evidence as to whether the measure is effective or not. Due to the nowadays low infection rates, studying of the importance and the effectiveness of each single element of the protocol is challenging: large cohorts of patients would be needed and each measure of these protocols would need to be studied “isolated” in randomized controlled trials (RCTs) to adequately assess their impact on the decline in infection rates. For ethical reasons alone, a study with, in extreme cases, only one prophylactic measure and omission of other known measures must be assessed critically. However, Bayston [14] considers this seeming lack of evidence acceptable in the light of the usual fall in infection rates after the implementation of a certain protocol.
One of the exceptions to this lack of evidence is the effectiveness of the use of antibiotic-impreganted catheters (AICs), which has now been proven by the BASICS study [5; 6]. Accordingly, the use of AICs is currently recommended in the IDSA's („Infectious Diseases Society of America's“) Clinical Practice Guidelines from 2017 [7] and the Hydrocephalus guidelines for pediatric patients (“Pediatric hydrocephalus: systematic literature review and evidence-based guidelines” [45]), which were updated in 2020 and now provide a so-called „Level 1 recommendation“ for their use [46].
The mode of action of such AICs and the existing evidence for their use is described in the following chapter.

With antibiotic-impregnated catheters (AICs), the antibiotics act in the area of the critical surgical wounds and directly on the surface of the implant. This is therefore a local application of antibiotics in which antibiotics are delivered to the surrounding tissue via the catheters for a certain period of time. Instead of an antimicrobial coating on the catheter surface, as it is sometimes done in other implants, “impregnation” means that the antibiotics are embedded in the silicone matrix of the catheter. The manufacture of such "impregnated" catheters utilizes the fact, that certain organic solvents are absorbed by the silicone, like water by a sponge. During drying, i.e. when the solvent escapes again from the silicone, the dissolved antibiotics remain in the catheter. From there, they are slowly released into the CSF and surrounding tissue over at least the critical first four weeks after implantation. This antibiotics-dispensing time can be determined through the production process. Compared to a single dose of antibiotics during surgery, this delivery over a certain critical period of time naturally represents a decisive advantage. A further advantage is that the antibiotic not only acts externally towards the surrounding tissue, but also into the inside catheter lumen filled with CSF.
The principle for such an impregnated catheter was already patented and described by Roger Bayston and Nancy Grove in 1987 (US Patent No. 4,917,686, see https://patents.google.com/patent/US4917686A/en) [47; 48]. The first commercial AICs, impregnated with Rifampicin (0.054 m%) in combination with Clindamycin Hydrochloride (0.15 m%) was Codman’s “Bactiseal”, mentioned by Alfred Aschoff already in 1999 [49]. There is, thus, already more than 20 years of clinical experience with catheters of this type.
The selection and concentration of the antibiotics used for impregnation of CSF diverting catheters must fulfill various boundary conditions. The antibiotics must not only be effective against the most important gram-positive pathogens (as shown in Figure 2 ), but must also be compatible with the silicone matrix. Only then can the release rate in the implanted state be precisely adjusted. Rifampicin is ideally suited in this respect.
As early as 2000, Kockro, Aschoff et al. [50] carried out a very impressive comparative study using an electron microscope (scanning electron microscopy, SEM), in which the fast time course of bacterial colonization and biofilm formation on a simple and a Rifampicin-impregnated catheter can be seen directly. Figure 3 shows how the growth of germs is almost completely suppressed on the AIC.

However, Rifampicin is unfortunately also known for its rapid development of resistance [51]. Therefore, it was combined with a second antibiotic (Clindamycin Hydrochloride), which has a different mode of action and thus makes the development of resistance unlikely. Another advantage of combining two different antibiotics is that individual concentrations can be lower and thus locally toxic concentrations can be avoided [52]
Since the first clinical study in 2003, Bactiseal has been used and described very often [53; 54]. In 2011, Medtronic additionally launched the ARES-catheter that conforms to the same specifications concerning the type and amount of antimicrobial agents used (510(k)-Nr.:K110560). Many study-data on the use of these products are available, which prove the safety and effectiveness of such AICs in the field of hydrocephalus therapy. Today, in Western countries, AICs are used as part of prophylactic protocols in a clear majority of cases. In their world-wide survey, Behbabani at el. analyzed 118 complete responses. The evaluation revealed that AICs are available in about 80% of the American and European institutions. Many other neurosurgical centers disclose that they routinely use AIC for all shunt or EVD procedures [25; 55; 56]. Data from two large multi-center studies also indicate the widespread use of AICs for pediatric patients in USA: the reported usage rates were over 50% within 2,007 cases, reported by Lakomkin et al. [57], and over 80% in 4,913 cases, as reported by Chu et al. [24].
Clinical Evidence for AICs: The BASICS trial
As mentioned above, strictly speaking there is a lack of clinical evidence for many individual prophylactic measures in a hygiene protocol, with exception of AICS. This is because in 2019, the efficacy of AICs was demonstrated for the first time and with a very high level of evidence in the BASICS study [5; 6]. The BASICS study is one of the few randomized, prospective, controlled trials (RCTs) with an excellent study design and valid case number planning. It is initially surprising that this evidence was only provided so late, i.e. only recently, as catheters of this type have been on the market for over two decades and a large number of studies on their use are available. However, this fact underlines how time-consuming, cost-intensive and difficult it is to conduct a proper, well-designed RCT. All previous studies, including prospective randomized studies, had too small a number of cases to prove efficacy.
The BASICS study, on the other hand, must be regarded as an outstanding first-class RCT, which is elaborate and proper in terms of design, implementation and evaluation. In particular, the final case number of 1,605 patients, which is very high compared to other studies, corresponds to a sufficient study power of at least 80%. It compares three treatment groups with each other, which is why it is also referred to as a 3-arm study: standard catheters, silver-impregnated catheters and AICs. Silver-impregnated catheters (SICs) theoretically have a similar mechanism of action to AICs, but use silver as an anti-microbial agent. As with AICs, the silver is located in the silicone matrix and is released into the surrounding tissue when implanted. SICs are described much less frequently than AICs in the literature and their effectiveness is controversial.
A total of 1,605 patients of all ages and hydrocephalus etiologies were studied in 21 UK and Irish hospitals between 2013 and 2017, i.e. more than 530 cases per group. Only patients who received a ventriculoperitoneal shunt (from any manufacturer) for the first time were included. Patients with subsequent shunts were excluded, as these are known to carry an increased risk of infection and therefore cannot be fairly compared with first-time shunts. After implantation, all patients were followed up for a minimum of 6 months and a maximum of 2 years.
As a result, a significant difference was found between the infection rate of 6% in the standard group and that of 2% in the AIC group. The easily overlooked but important statement that this reduction was "significant" means that this difference was almost certainly not due to chance - "due to normal unavoidable variability" – but was actually due to the effectiveness of the AICs. As an important result of the BASICS study, it should be emphasized here that the group with the silver catheters had the same infection rate as the standard group (6%), so that it cannot be assumed that the SICs are effective.
The extensive BASICS data was also thoroughly analyzed in terms of the costs incurred. The result was that the use of AICs leads to an effective cost saving (in the UK healthcare system) of £135,753 (approx. $171,000, approx. €158,000) per infection avoided. This figure corresponds well with the results of previous comprehensive cost/benefit analyses. In 2015, Parker et al. analyzed data from 287 US hospitals from 10,819 adult and 1,770 pediatric patients [59]. They estimate the total additional costs per infection at approx. 45,000-95,000$ for adults and approx. 56,000-121,000$ for children.
„In analysis of this large, nationwide database, AICs were found to be associated with a significant reduction in infection incidence, resulting in tremendous cost savings. AICs were associated with a cost savings of $42,125 and $230,390 per 100 de novo shunts placed in adult and pediatric patients, respectively.“
Also in 2015, Edwards et al. [34] presented a comprehensive literature analysis, which came to the following conclusion:
“The rate of decrease in infection with AIC shunts was shown to have the greatest impact on the cost savings realized with use of AIC shunts.”
In terms of the cost-benefit analysis, the BASICS study has therefore fully confirmed the earlier analyses and, despite the higher initial costs, a relevant cost saving through the use of AICs can now be considered certain.
The decrease in the likelihood of infection with age was also confirmed, whereby the effectiveness of AICs is most evident in children and those with high infection rates.
However, BASICS also came to an unexpected conclusion: the cumulative overall revision rates, which include all possible complications or shunt failures, remained just as high for AICs as for standard catheters (and silver catheters). In other words, the mean lifetime of the shunt did not increase with AICs despite a reduction in the risk of infection. In the AIC study group, other complications instead of infections occurred more frequently, so that the relative number of all revisions remained unchanged. In particular, blockages of the ventricular catheter and valves increased. Mallucci et al. hypothesized that the AICs convert an infection into a low-grade infection in which the original pathogens with low virulence are confined to a biofilm in the (non-impregnated) valve body. This could then lead to mechanical failure. The authors write:
“However, changes in CSF composition and flow (such as debris or high protein) might block the intricate valve mechanism. Our study was not powered or designed to answer this question directly,[...] Nevertheless, from the patient’s perspective, although mechanical shunt revision still requires surgery which could impact on quality of life, the hospital admission is short, prolonged antibiotics are not required, and patients recover faster with fewer long-term neurological sequelae than if their shunts become infected.”
Clearly, a revision due to an infection is a worse complication than a revision caused by other reasons, as these infections are more difficult to treat and increase the risk of subsequent infections. Long antibiotic treatments and reoperations are not only devastating for the patient but also present a great burden to the healthcare system due to costs of the treatment (antibiotics, multiple reoperations) and prolonged hospital stays.
If the occurrence of increased revision rates for non-infectious reasons when using AICs is indeed confirmed, the underlying cause must be clarified in the future.
We end our overview with a quote from another recent meta-analysis, which also takes a critical look at the results of the BASICS study, the study by Goda et al. [60]. This work should not go unmentioned here because of the application of various, rather rarely used complex methods (“Cochrane risk of bias assessment tool”, “trial sequential analysis”, “network meta-analysis”) to clarify the evidence question:
“…antibiotic medicated ventriculoperitoneal shunts had the highest probability of being the best option in terms of the relative infection rates.”
To date, the available evidence, thus, points to the effectiveness and cost-efficiency of antibiotic-impregnated catheters, giving surgeons a well-studied option to address infection risks in shunt surgery.

Shunt infections are a common and serious complication of shunt treatment. They massively affect the quality of life of patients and lead to relevant costs for the healthcare system. Most infections occur in the first month after surgery, suggesting that contamination of the implant most likely happens during surgery. Surgical implant infections are often hard to treat, as the bacteria commonly involved in these infections are able to produce biofilms, which effectively protect them against host immune responses and drug treatments. Infected shunts thus have to be removed and the patient treated with a course of antibiotics before a new shunt can be implanted.
The prevention of infections and their difficult and burdensome treatment through prophylactic hygiene measures is therefore of the utmost importance.
A wealth of data shows that there are prophylactic measures that can be taken to successfully reduce shunt infections. The best results have been achieved with bundles of different measures, including common sense aseptic techniques, preoperative patient preparation, the scheduling and timing of shunt surgeries, intraoperative measures such as double gloving and “no-touch” methods as well as antibiotic prophylaxis. As local applications of antibiotics, antibiotic-impregnated catheters (AIC) have quickly become a widely-used preventive measure. Clinical studies have shown a reduction in shunt infection in comparison to other catheters and demonstrated their cost-effectiveness. AIC are particularly beneficial for those patients at higher risk of infection, namely children, (premature) newborns, patients with recurring infections, or those who received a treatment with EVD before.
Nowadays, the use of AICs can be considered state-of-the-art and is accordingly recommended in several guidelines.
Literature
1. Darouiche RO. Antimicrobial approaches for preventing infections associated with surgical implants. Clin Infect Dis. 2003 May 15;36(10):1284-9. doi: 10.1086/374842. Epub 2003 May 9.
2. Isaacs AM, Yang R, Cadieux M, Ben-Israel D, Sader N, Opoku-Darko M, Frizon L, Yong H, Premji Z, Nagel S, Hamilton MG; Adult Hydrocephalus Clinical Research Network (AHCRN); Other Adult Hydrocephalus Clinical Research Network Site Principal Investigators. Characteristics of shunt failure in 38,095 adult shunt insertion surgeries: a systematic review and meta-analysis. Neurosurg Focus. 2023 Apr;54(4):E2. doi: 10.3171/2023.1.FOCUS22637.
3. Hauptman, J.S., Lutz, B.R., Hanak, B.W., Browd, S.R. (2019). Technical Advances in the Treatment of Hydrocephalus: Current and Future State. In: Limbrick Jr., D., Leonard, J. (eds) Cerebrospinal Fluid Disorders . Springer, Cham. doi.org/10.1007/978-3-319-97928-1_21.
4. Borgbjerg BM, Gjerris F, Albeck MJ, Børgesen SE. Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first-time shunts. Acta Neurochir (Wien). 1995;136(1-2):1-7. doi: 10.1007/BF01411427.
5. Mallucci CL, Jenkinson MD, Conroy EJ, Hartley JC, Brown M, Dalton J, Kearns T, Moitt T, Griffiths MJ, Culeddu G, Solomon T, Hughes D, Gamble C; BASICS Study collaborators. Antibiotic or silver versus standard ventriculoperitoneal shunts (BASICS): a multicentre, single-blinded, randomised trial and economic evaluation. Lancet. 2019 Oct 26;394(10208):1530-1539. doi: 10.1016/S0140-6736(19)31603-4. Epub 2019 Sep 12. Erratum in: Lancet. 2019 Sep 18;: Erratum in: Lancet. 2020 Jun 13;395(10240):1834.
6. Mallucci CL, Jenkinson MD, Conroy EJ, Hartley JC, Brown M, Moitt T, Dalton J, Kearns T, Griffiths MJ, Culeddu G, Solomon T, Hughes D, Gamble C; BASICS study collaborators. Silver-impregnated, antibiotic-impregnated or non-impregnated ventriculoperitoneal shunts to prevent shunt infection: the BASICS three-arm RCT. Health Technol Assess. 2020 Mar;24(17):1-114. doi: 10.3310/hta24170.
7. Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, van de Beek D, Bleck TP, Garton HJL, Zunt JR. 2017 Infectious Diseases Society of America's Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis. 2017 Mar 15;64(6):e34-e65. doi: 10.1093/cid/ciw861.
8. Bock HC, Kanzler M, Thomale UW, Ludwig HC. Implementing a digital real-time Hydrocephalus and Shunt Registry to evaluate contemporary pattern of care and surgical outcome in pediatric hydrocephalus. Childs Nerv Syst. 2018 Mar;34(3):457-464. doi: 10.1007/s00381-017-3654-0. Epub 2017 Nov 9.
9. Fernández-Méndez R, Richards HK, Seeley HM, Pickard JD, Joannides AJ; UKSR collaborators. Current epidemiology of cerebrospinal fluid shunt surgery in the UK and Ireland (2004-2013). J Neurol Neurosurg Psychiatry. 2019 Jul;90(7):747-754. doi: 10.1136/jnnp-2018-319927. Epub 2019 Mar 25.
10. Pickard JD, Richards H, Joannides A (2017) UK Shunt Registry Draft Report:2017
11. George R, Leibrock L, Epstein M. Long-term analysis of cerebrospinal fluid shunt infections. A 25-year experience. J Neurosurg. 1979 Dec;51(6):804-11. doi: 10.3171/jns.1979.51.6.0804.
12. Michelle Paff, Daniela Alexandru-Abrams, Michael Muhonen, William Loudon, Ventriculoperitoneal shunt complications: A review, Interdisciplinary Neurosurgery, Volume 13, 2018, Pages 66-70, ISSN 2214-7519, doi.org/10.1016/j.inat.2018.04.004.
13. Hommelstad J, Madsø A, Eide PK. Significant reduction of shunt infection rate in children below 1 year of age after implementation of a perioperative protocol. Acta Neurochir (Wien). 2013 Mar;155(3):523-31. doi: 10.1007/s00701-012-1574-z. Epub 2012 Dec 8.
14. Bayston, R. (2021). Infections in CSF Shunts and External Ventricular Drainage. In P. K. Bektaşoğlu & B. Gürer (Eds.), Cerebrospinal Fluid. IntechOpen. doi.org/10.5772/intechopen.98910
15. Sarmey N, Kshettry VR, Shriver MF, Habboub G, Machado AG, Weil RJ. Evidence-based interventions to reduce shunt infections: a systematic review. Childs Nerv Syst. 2015 Apr;31(4):541-9. doi: 10.1007/s00381-015-2637-2. Epub 2015 Feb 17.
16. Vinchon M, Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst. 2006 Jul;22(7):692-7. doi: 10.1007/s00381-005-0037-8. Epub 2006 Mar 14.
17. Choux M, Genitori L, Lang D, Lena G. Shunt implantation: reducing the incidence of shunt infection. J Neurosurg. 1992 Dec;77(6):875-80. doi: 10.3171/jns.1992.77.6.0875.
18. Cochrane DD, Kestle JR. The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. Pediatr Neurosurg. 2003 Jun;38(6):295-301. doi: 10.1159/000070413.
19. Prusseit J, Simon M, von der Brelie C, Heep A, Molitor E, Volz S, Simon A. Epidemiology, prevention and management of ventriculoperitoneal shunt infections in children. Pediatr Neurosurg. 2009;45(5):325-36. doi: 10.1159/000257520. Epub 2009 Nov 11.
20. Simon TD, Butler J, Whitlock KB, Browd SR, Holubkov R, Kestle JR, Kulkarni AV, Langley M, Limbrick DD Jr, Mayer-Hamblett N, Tamber M, Wellons JC 3rd, Whitehead WE, Riva-Cambrin J; Hydrocephalus Clinical Research Network. Risk factors for first cerebrospinal fluid shunt infection: findings from a multi-center prospective cohort study. J Pediatr. 2014 Jun;164(6):1462-8.e2. doi: 10.1016/j.jpeds.2014.02.013. Epub 2014 Mar 21.
21. Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, Lafleur B, Dean JM, Kestle JR; Hydrocephalus Clinical Research Network. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. J Neurosurg Pediatr. 2009 Aug;4(2):156-65. doi: 10.3171/2009.3.PEDS08215.
22. Behbahani M, Khalid SI, Lam SK, Caceres A. Global trends in the evaluation and management of cerebrospinal fluid shunt infection: a cooperative ISPN survey. Childs Nerv Syst. 2020 Dec;36(12):2949-2960. doi: 10.1007/s00381-020-04699-z. Epub 2020 Jun 9.
23. Okamura Y, Maruyama K, Fukuda S, Horikawa H, Sasaki N, Noguchi A, Nagane M, Shiokawa Y. Detailed standardized protocol to prevent cerebrospinal fluid shunt infection. J Neurosurg. 2019 Feb 15;132(3):755-759. doi: 10.3171/2018.10.JNS181432.
24. Chu J, Jensen H, Holubkov R, Krieger MD, Kulkarni AV, Riva-Cambrin J, Rozzelle CJ, Limbrick DD, Wellons JC, Browd SR, Whitehead WE, Pollack IF, Simon TD, Tamber MS, Hauptman JS, Pindrik J, Naftel RP, McDonald PJ, Hankinson TC, Jackson EM, Rocque BG, Reeder R, Drake JM, Kestle JRW; Hydrocephalus Clinical Research Network; Hydrocephalus Clinical Research Network Members. The Hydrocephalus Clinical Research Network quality improvement initiative: the role of antibiotic-impregnated catheters and vancomycin wound irrigation. J Neurosurg Pediatr. 2022 Mar 18;29(6):711-718. doi: 10.3171/2022.2.PEDS2214.
25. Shibamura-Fujiogi M, Ormsby J, Breibart M, Warf B, Priebe GP, Soriano SG, Sandora TJ, Yuki K. Risk factors for pediatric surgical site infection following neurosurgical procedures for hydrocephalus: a retrospective single-center cohort study. BMC Anesthesiol. 2021 Apr 21;21(1):124. doi: 10.1186/s12871-021-01342-5.
26. Wells DL, Allen JM. Ventriculoperitoneal shunt infections in adult patients. AACN Adv Crit Care. 2013 Jan-Mar;24(1):6-12; quiz 13-4. doi: 10.1097/NCI.0b013e31827be1d1.
27. Arciola CR, Campoccia D, Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018 Jul;16(7):397-409. doi: 10.1038/s41579-018-0019-y.
28. Bayston R, Penny SR. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonisation of Holter shunts. Dev Med Child Neurol Suppl. 1972;27:25-8. doi: 10.1111/j.1469-8749.1972.tb09769.x.
29. Zhang K, Li X, Yu C, Wang Y. Promising Therapeutic Strategies Against Microbial Biofilm Challenges. Front Cell Infect Microbiol. 2020 Jul 28;10:359. doi: 10.3389/fcimb.2020.00359.
30. Gilbert P, Collier PJ, Brown MR. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob Agents Chemother. 1990 Oct;34(10):1865-8. doi: 10.1128/AAC.34.10.1865.
31. Schierholz JM, Beuth J. Implant infections: a haven for opportunistic bacteria. J Hosp Infect. 2001 Oct;49(2):87-93. doi: 10.1053/jhin.2001.1052.
32. Conen A, Raabe A, Schaller K, Fux CA, Vajkoczy P, Trampuz A. Management of neurosurgical implant-associated infections. Swiss Med Wkly. 2020 Apr 24;150:w20208. doi: 10.4414/smw.2020.20208.
33. James HE, Bradley JS. Aggressive management of shunt infection: combined intravenous and intraventricular antibiotic therapy for twelve or less days. Pediatr Neurosurg. 2008;44(2):104-11. doi: 10.1159/000113111. Epub 2008 Jan 24.
34. Edwards NC, Engelhart L, Casamento EM, McGirt MJ. Cost-consequence analysis of antibiotic-impregnated shunts and external ventricular drains in hydrocephalus. J Neurosurg. 2015 Jan;122(1):139-47. doi: 10.3171/2014.9.JNS131277.
35. Arthur AS, Whitehead WE, Kestle JR. Duration of antibiotic therapy for the treatment of shunt infection: a surgeon and patient survey. Pediatr Neurosurg. 2002 May;36(5):256-9. doi: 10.1159/000058429.
36. Pirotte BJ, Lubansu A, Bruneau M, Loqa C, Van Cutsem N, Brotchi J. Sterile surgical technique for shunt placement reduces the shunt infection rate in children: preliminary analysis of a prospective protocol in 115 consecutive procedures. Childs Nerv Syst. 2007 Nov;23(11):1251-61. doi: 10.1007/s00381-007-0415-5. Epub 2007 Aug 18.
37. Kestle JR, Riva-Cambrin J, Wellons JC 3rd, Kulkarni AV, Whitehead WE, Walker ML, Oakes WJ, Drake JM, Luerssen TG, Simon TD, Holubkov R; Hydrocephalus Clinical Research Network. A standardized protocol to reduce cerebrospinal fluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative. J Neurosurg Pediatr. 2011 Jul;8(1):22-9. doi: 10.3171/2011.4.PEDS10551.
38. Kestle JR, Holubkov R, Douglas Cochrane D, Kulkarni AV, Limbrick DD Jr, Luerssen TG, Jerry Oakes W, Riva-Cambrin J, Rozzelle C, Simon TD, Walker ML, Wellons JC 3rd, Browd SR, Drake JM, Shannon CN, Tamber MS, Whitehead WE; Hydrocephalus Clinical Research Network. A new Hydrocephalus Clinical Research Network protocol to reduce cerebrospinal fluid shunt infection. J Neurosurg Pediatr. 2016 Apr;17(4):391-6. doi: 10.3171/2015.8.PEDS15253. Epub 2015 Dec 18.
39. Faillace WJ. A no-touch technique protocol to diminish cerebrospinal fluid shunt infection. Surg Neurol. 1995 Apr;43(4):344-50. doi: 10.1016/0090-3019(95)80060-t.
40. Rehman AU, Rehman TU, Bashir HH, Gupta V. A simple method to reduce infection of ventriculoperitoneal shunts. J Neurosurg Pediatr. 2010 Jun;5(6):569-72. doi: 10.3171/2010.2.PEDS09151.
41. Tulipan N, Cleves MA. Effect of an intraoperative double-gloving strategy on the incidence of cerebrospinal fluid shunt infection. J Neurosurg. 2006 Jan;104(1 Suppl):5-8. doi: 10.3171/ped.2006.104.1.5.
42. Rozzelle CJ, Leonardo J, Li V. Antimicrobial suture wound closure for cerebrospinal fluid shunt surgery: a prospective, double-blinded, randomized controlled trial. J Neurosurg Pediatr. 2008 Aug;2(2):111-7. doi: 10.3171/PED/2008/2/8/111.
43. Haliasos N, Bhatia R, Hartley J, Thompson D. Ioban drapes against shunt infections? Childs Nerv Syst. 2012 Apr;28(4):509-10. doi: 10.1007/s00381-012-1724-x. Epub 2012 Feb 22.
44. Klimo P Jr, Flannery AM. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 6: Preoperative antibiotics for shunt surgery in children with hydrocephalus: a systematic review and meta-analysis. J Neurosurg Pediatr. 2015 Aug;16(2):237-9. doi: 10.3171/2014.7.PEDS14326a. Epub 2015 May 8. Erratum for: J Neurosurg Pediatr. 2014 Nov;14 Suppl 1:44-52.
45. Klimo P Jr, Thompson CJ, Baird LC, Flannery AM; Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 7: Antibiotic-impregnated shunt systems versus conventional shunts in children: a systematic review and meta-analysis. J Neurosurg Pediatr. 2014 Nov;14 Suppl 1:53-9. doi: 10.3171/2014.7.PEDS14327.
46. Bauer DF, Baird LC, Klimo P, Mazzola CA, Nikas DC, Tamber MS, Flannery AM. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Treatment of Pediatric Hydrocephalus: Update of the 2014 Guidelines. Neurosurgery. 2020 Nov 16;87(6):1071-1075. doi: 10.1093/neuros/nyaa434.
47. Bayston R, Grove N, Siegel J, Lawellin D, Barsham S. Prevention of hydrocephalus shunt catheter colonisation in vitro by impregnation with antimicrobials. J Neurol Neurosurg Psychiatry. 1989 May;52(5):605-9. doi: 10.1136/jnnp.52.5.605.
48. Patent: Bayston R. and Grove N. J., Antimicrobial Device and Method, No. 1987,
49. Aschoff A, Oikonomou J, Hashemi B, Schulte C, Kremer P, Wabel P, Leonhardt S, Kunze St. 482 Hydrocephalus Valves Tested in Vitro and a Review on 652 Tests Reported in Literature. Journal (Issue), 1999 Jan
50. Kockro RA, Hampl JA, Jansen B, Peters G, Scheihing M, Giacomelli R, Kunze S, Aschoff A. Use of scanning electron microscopy to investigate the prophylactic efficacy of rifampin-impregnated CSF shunt catheters. J Med Microbiol. 2000 May;49(5):441-450. doi: 10.1099/0022-1317-49-5-441.
51. Sköld O. Antibiotics and antibiotic resistance. John Wiley & Sons, Inc. 2011 Sep; doi: 10.1002/9781118075609.
52. Bayston R, Lambert E. Duration of protective activity of cerebrospinal fluid shunt catheters impregnated with antimicrobial agents to prevent bacterial catheter-related infection. J Neurosurg. 1997 Aug;87(2):247-51. doi: 10.3171/jns.1997.87.2.0247.
53. Govender ST, Nathoo N, van Dellen JR. Evaluation of an antibiotic-impregnated shunt system for the treatment of hydrocephalus. J Neurosurg. 2003 Nov;99(5):831-9. doi: 10.3171/jns.2003.99.5.0831.
54. Aryan HE, Meltzer HS, Park MS, Bennett RL, Jandial R, Levy ML. Initial experience with antibiotic-impregnated silicone catheters for shunting of cerebrospinal fluid in children. Childs Nerv Syst. 2005 Jan;21(1):56-61. doi: 10.1007/s00381-004-1052-x. Epub 2004 Oct 12.
55. Schaumann A, Bührer C, Schulz M, Thomale UW. Neuroendoscopic surgery in neonates - indication and results over a 10-year practice. Childs Nerv Syst. 2021 Nov;37(11):3541-3548. doi: 10.1007/s00381-021-05272-y. Epub 2021 Jul 3.
56. Sweid A, Weinberg JH, Abbas R, El Naamani K, Tjoumakaris S, Wamsley C, Mann EJ, Neely C, Head J, Nauheim D, Hauge J, Gooch MR, Herial N, Zarzour H, Alexander TD, Missios S, Hasan D, Chalouhi N, Harrop J, Rosenwasser RH, Jabbour P. Predictors of ventriculostomy infection in a large single-center cohort. J Neurosurg. 2020 Apr 10;134(3):1218-1225. doi: 10.3171/2020.2.JNS192051.
57. Lakomkin N, Hadjipanayis CG. The Role of Prophylactic Intraventricular Antibiotics in Reducing the Incidence of Infection and Revision Surgery in Pediatric Patients Undergoing Shunt Placement. Neurosurgery. 2021 Jan 13;88(2):301-305. doi: 10.1093/neuros/nyaa413. Erratum in: Neurosurgery. 2021 Apr 15;88(5):1042.
58. Mbabazi-Kabachelor E, Shah M, Vaughan KA, Mugamba J, Ssenyonga P, Onen J, Nalule E, Kapur K, Warf BC. Infection risk for Bactiseal Universal Shunts versus Chhabra shunts in Ugandan infants: a randomized controlled trial. J Neurosurg Pediatr. 2019 Jan 4;23(3):397-406. doi: 10.3171/2018.10.PEDS18354.
59. Parker SL, McGirt MJ, Murphy JA, Megerian JT, Stout M, Engelhart L. Cost savings associated with antibiotic-impregnated shunt catheters in the treatment of adult and pediatric hydrocephalus. World Neurosurg. 2015 Mar;83(3):382-6. doi: 10.1016/j.wneu.2014.06.010. Epub 2014 Jun 13.
60. Goda R, Ganeshkumar A, Katiyar V, Sharma R, Gurjar HK, Chaturvedi A, Sahu R, Rai HIS, Vora Z. Efficacy of antimicrobial medicated ventricular catheters: a network meta-analysis with trial sequential analysis. Neurosurg Rev. 2022 Feb;45(1):91-102. doi: 10.1007/s10143-021-01532-2. Epub 2021 May 19.
